There is a very important metric of brain competence that stands alongside of IQ and executive function in determining what a person’s brain will be capable of over its lifetime. The excitation/inhibition ratio, or the E/I balance, results from the constant tug-of-war between the brain’s excitatory neurons and neurotransmitters (glutamate), and its inhibitory neurons and neurotransmitters (GABA).
As a child grows into adulthood, the E/I balance of his brain will be reduced, reflecting the growing power of inhibitory networks to keep the excitatory networks under control.
The E/I ratio of children decreases with healthy development. Children with a lower E/I ratio were observed to have better performance than their peers in cognitive tests such as memory and intelligence, according to studies by researchers from the Centre for Sleep and Cognition at the Yong Loo Lin School of Medicine (NUS Medicine). __ Source … Original Study
If the E/I balance is off, a number of neurodevelopmental, neuropsychiatric, and neurodegenerative diseases can result, such as autism, schizophrenia, and dementia. Even a very intelligent person in the prime of life can find himself held back from his potential if his E/I balance cannot support the goals he has set for his life.
The E/I balance arises from very low-level activity over all parts of the brain, particularly the cortex. It can be measured using MEG/EEG, fMRI, or a combination of the approaches.
A Closer Look at Hyperexcitability and Alzheimer’s Disease
Alzheimer’s disease (AD) is arguably the most common cause of dementia in the elderly and is marked by progressive synaptic degeneration, which in turn leads to cognitive decline. Studies in patients and in various AD models have shown that one of the early signatures of AD is neuronal hyperactivity. This excessive electrical activity contributes to dysregulated neural network function and synaptic damage. Mechanistically, evidence suggests that hyperexcitability accelerates production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that contribute to neural network impairment and synapse loss. This review focuses on the pathways and molecular changes that cause hyperexcitability and how RNS-dependent posttranslational modifications, represented predominantly by protein S-nitrosylation, mediate, at least in part, the deleterious effects of hyperexcitability on single neurons and the neural network, resulting in synaptic loss in AD. __ Frontiers in Neural Circuits 2023
Research suggests that brain cells can be lost at an accelerated rate when the brain operates under conditions of hyperexcitability. This is a problem for people of all ages, but it comes to a head as a person approaches the last decades of his life. Alzheimer’s and other dementias can be a result. In early years, autism can result from an E/I imbalance. And in adolescence and middle years, a variety of problems — including schizophrenia and a wide range of other problems — can emerge.
I can hear a lot of yawning out there, but you should remember that one of the things that makes the difference between being able to yawn and then go about your business — and only being able to sit and drool with a vacant look on your face — is the E/I balance.
Fortunately, there may soon be safe ways to tweak the E/I balance to make your brain more powerful, more healthy, and more resilient to inadvertent damage such as stroke, infection, or chemotoxicity.
The Lipton Lab at Scripps in La Jolla, CA, is working on different chemotherapeutics which may help some conditions involving altered E/I balance:
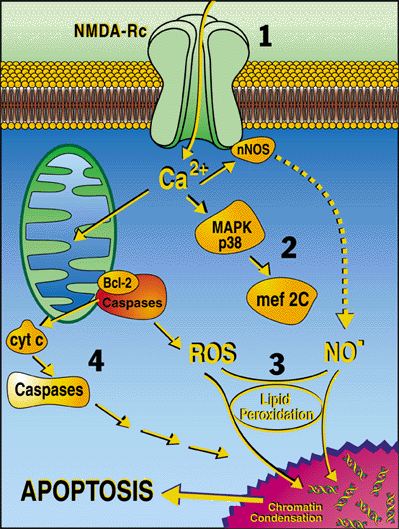
Our laboratory uses basic molecular signaling pathways to protect synapses and prevent neuronal injury in normal aging and various neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, stroke (Vascular dementia), and other forms of dementia. Neuronal synaptic damage is curtailed by preventing excessive activity of the NMDA subtype of glutamate receptor and its downstream effectors (see figure and legend).
The laboratory is best known for discovering the mechanism of action and contributing to the clinical development of the FDA-approved Alzheimer’s drug, memantine (Namenda®, NamendaXR®, Namzaric®), and for co-discovering the posttranslational redox modification, S-nitrosylation. Recently, we combined memantine with S-nitrosylation chemistry to produce a new drug called NitroSynapsin, which displays disease-modifying activity in animal models of Alzheimer’s disease, both protecting synapses and improving neurobehavioral deficits.
Our group also characterized HIV-related pathways to neuronal damage, discovered the NR3 (now known at GluN3) family of modulatory NMDA-type glutamate receptor subunits in the brain, characterized the molecular pathways for protecting neurons with Erythropoietin, and discovered the transcription factor MEF2C. Members of the group showed that MEF2C activity is regulated by S-nitrosylation and serves as a master switch for neurogenesis from human neural stem cells. Dysregulated MEF2C is involved in the pathogenesis of Parkinson’s disease, Alzheimer’s disease, Autism-Spectrum Disorder, and Vascular dementia.
Ongoing research in the lab uses “disease-in-a-dish” models generated from patient-derived human induced pluripotent stem cells (hiPSCs) in 2D cultures and 3D-brain organoids (“mini-brains”) as well as in transgenic mouse models. These models of neurodegenerative and neurodevelopmental disease are used to study aberrant redox/S-nitrosylation pathways to synaptic damage. Using these approaches, the laboratory is developing novel drugs to combat Alzheimer’s disease (AD), Parkinson’s disease (PD), Vascular dementia (VaD), and other neurodegenerative disorders, as well as Autism-Spectrum Disorder (ASD) and Intellectual and Developmental Disability (IDD). Tissue culture models complement whole-animal approaches. A plethora of techniques are employed including chemical biology, molecular biology, patch-clamp electrophysiology, calcium imaging, and neurobehavioral paradigms. __ Lipton Lab
More on NitroSynapsin:
Lipton and his team were able to show that NitroSynapsin not only prevents the loss of human brain synapses but promotes the regrowth of lost synapses. They tested the drug both on mice and on 3-D mini-brains, also known as cerebral organoids, that are created using skin cells from Alzheimer’s patients.
“We were able to show, for the first time, that the drug works in a human context,” says Lipton, who is also the director of Translational Neuroscience and the Hannah and Eugene Step Chair at Scripps Research, and a practicing clinical neurologist. “This is a significant finding, as experimental drugs for Alzheimer’s and other progressive brain diseases have an unfortunate track record of working well in mice, then failing when tested in people.”
… The study showed that the small clumps of amyloid beta protein that were thought to injure synapses directly actually induce the release of excessive amounts of the neurotransmitter glutamate. In this case, glutamate is released from brain cells called astrocytes, which are adjacent to nerve cells. Normal levels of glutamate promote memory and learning, but excessive levels are harmful to synapses. In patients with Alzheimer’s, excessive glutamate contributes to the dangerous level of electrical activity in the brain, resulting in synapse loss. __ Lipton Neurosynaptin Study in Alzheimer’s
The use of pharmaceuticals in disease states such as stroke, heart disease, Alzheimer’s, schizophrenia, etc. is widely accepted. But use of drugs in persons who are not clearly suffering from a well-defined disease state can be highly controversial.
And yet for those who understand the physiological basis of sub-optimal E/I balance — and the unfortunate effects such an imbalance can have on a person’s life even if he is not diagnosed with a disease — a trial of relatively safe drugs with tolerable side effects might be attempted in certain situations. There is still a lot to learn about the chemistry, physiology, pathology, and the potential pharmacology involved in dealing with the wide range of conditions affected by E/I imbalance.
Remember: A person can be very intelligent, but because his E/I balance is sub-optimal he may be performing far below his potential. From the standpoint of human societies, there is a lot of potential being wasted.